Coronavirus
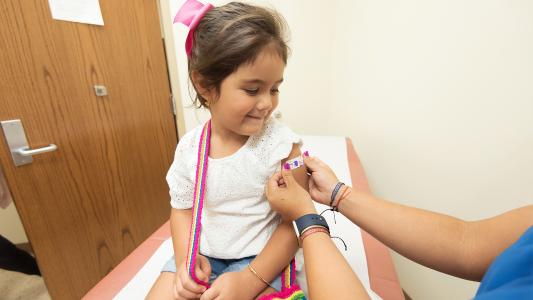
FDA panel recommends COVID-19 vaccine for 5 to 11 year olds (Updated)
An FDA panel voted to authorize Pfizer’s COVID-19 vaccine for kids ages 5 to 11. Here’s what you need to know about it.
Ear sensor can monitor COVID-19 patients remotely
An ear sensor successfully alerted doctors to signs that COVID-19 patients isolating at home were getting worse and should be hospitalized.
Pill to treat COVID-19 cuts risk of hospitalization or death in half
A pill to treat COVID-19 that cut hospitalizations and deaths in half could become the first oral medication against the disease.
Antiviral reduces COVID-19 hospitalizations by 87%
The FDA-approved antiviral drug remdesivir prevents high-risk people from ending up in the hospital, if given early.
FDA approves COVID-19 boosters for seniors, high-risk groups (Updated)
The FDA has expanded its authorization of COVID-19 boosters of Pfizer’s vaccine to include seniors and people in high-risk populations.
Pfizer says its COVID-19 vaccine works in kids
New trial results suggest Pfizer’s COVID-19 vaccine works in kids between the ages of 5 and 11, safely triggering a robust antibody response.
Moderna’s new vaccine targets COVID-19, the flu, and RSV
Moderna is developing a combination vaccine to protect against COVID-19, seasonal influenza, and respiratory syncytial virus.
Study: COVID-19 booster shots dramatically reduce infection risk
A large COVID-19 booster shot study in Israel found that a third dose significantly increased seniors’ protection against the coronavirus.
UK researchers are growing the Delta variant for human challenge trials
Responding to the variant’s rise, UK researchers are growing the Delta variant for trials.
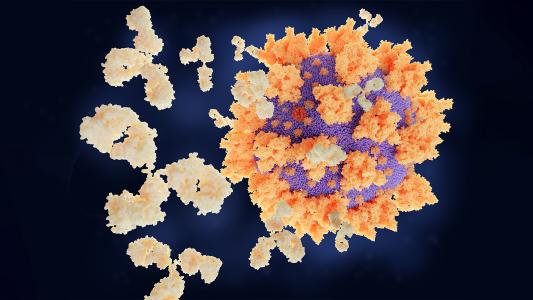
One antibody stops all strains of COVID-19 from infecting cells
A newly discovered antibody can neutralize all strains of COVID-19 and every other sarbecovirus known to infect humans.