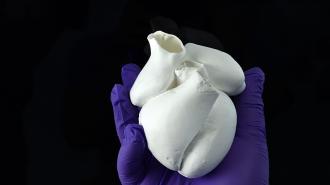
Researchers at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS) believe they have taken a step towards solving a centuries-old heart muscle mystery — and towards the ability to eventually fabricate entire human hearts for transplantation.
Using a new form of textile manufacturing, akin to a cotton candy machine, the SEAS team was able to build a model of human ventricles (those beefy chambers in the heart which pump blood out into the body) that were not only capable of beating, but recreated the unique structure of heart muscles — a structure that scientists have been trying to tease out the meaning of since 1669.
“This work is a major step forward for organ biofabrication and brings us closer to our ultimate goal of building a human heart for transplant,” Kit Parker, the study’s senior author and SEAS professor of bioengineering and applied physics, said.
Heart muscle isn’t laid down in neat lines like a French mime’s shirt. Instead, it forms something of a spiral, the definitive purpose of which has been a mystery to science since the 17th century.
A broken heart: The ability to bioprint a human heart is one of the Holy Grails of cardiac medicine, since the heart — unlike many other organs in the body — does not repair itself after suffering an injury, like a heart attack.
Recent research involving gene therapy and microRNA technology has allowed scientists to regenerate heart muscle in pig hearts, a close model for humans, while other research groups are still racing to be able to build hearts for transplantation.
To do that, however, we need to be able to mimic the structures of the heart. That includes a structural feature called “helical alignment” that researchers have been puzzling over since the 17th century.
A heart muscle mystery: Heart muscle isn’t laid down in neat lines like a French mime’s shirt; instead, it forms something of a spiral. This helical alignment leads to a wringing-like motion when it pumps.
The spiraling alignment was first characterized by the English physician Richard Lower in 1669 in his book Tractatus de Corde — a real work of the heart, literally. The pioneering cardiovascular researcher didn’t know exactly why heart muscle spiraled, though, and 300 years of research had yet to find the definitive answer.
In a nice bit of poetic symmetry, in 1969, Edward Sallin of the University of Alabama Birmingham Medical School put forth a theory: that the spiraling heart muscle helps the heart pump more blood with each beat, making it the most efficient heart design.
So why don’t we know if Sallin’s right, fifty years later?
“Testing this possibility is difficult, however, because it is challenging to reproduce the fine spatial features and complex structures of the heart’s musculature using current techniques,” the SEAS researchers wrote in their study, published in Science.
The Harvard team set out to make it happen.
“Our goal was to build a model where we could test Sallin’s hypothesis and study the relative importance of the heart’s helical structure,” SEAS postdoc fellow and study co-first author John Zimmerman said.
Cardiac cotton candy: The SEAS team used a recently developed process from Parker’s lab, called Focused Rotary Jet Spinning (FRJS), to make their heart model.
The researchers describe FRJS as similar to a cotton candy machine. The device spins, forcing out a polymer that can be adjusted by using an airstream. By angling and rotating it, the team found they could form scaffolding that could recreate heart muscle’s spirals.
“The human heart actually has multiple layers of helically aligned muscles with different angles of alignment,” Huibin Chang, SEAS postdoctoral fellow and co-first author, said. “With FRJS, we can recreate those complex structures in a really precise way, forming single and even four chambered ventricle structures.”
The team created spiral scaffolding in the shape of ventricles, then rat and human heart muscle cells were then spread on, like a cardiac chia pet, growing multiple thin layers of beating muscle tissue.
Using their new technique, “we can recreate those complex structures [of the heart] in a really precise way, forming single and even four chambered ventricle structures.”
Huibin Chang
The model ventricles beat with the same wringing motion as a real human heart, which the team found did a better job of pumping blood out of the ventricle — a measurement known as ejection fraction — supporting Sallin’s theory.
The researchers were able to use FRJS to make larger heart models, from a human to a Mine whale, although they did not cover them in the billions of cells they’d need to grow beating tissue.
In addition to providing a model of the spiraling heart muscle to study, the researchers believe their work can help improve the biofabrication of hearts for transplants, and even have uses beyond medicine, like food packaging.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.