The first-in-human trial of FLASH radiotherapy found the experimental treatment to be safe and effective — suggesting that there may be a faster, less painful way to use radiation against cancer.
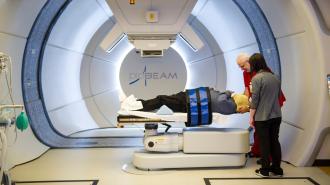
The status quo: Radiation therapy is a common cancer treatment that uses high doses of radiation to kill or slow the growth of cancer cells. Usually, this is done by aiming a beam of radiation directly at a tumor for a few minutes. This part of the process is painless, like getting an X-ray.
Patients typically undergo daily treatments five days a week for several weeks, and including setup time, a treatment usually takes about 15 to 30 minutes.
With traditional radiation therapy, dosages may have to be limited to avoid painful side effects.
The challenge: By shrinking a tumor, radiation therapy can not only fight cancer, but also potentially relieve patients’ pain or other symptoms caused by it. But the beam of radiation can damage healthy tissue near the tumor, too, causing pain and other side effects.
To minimize these adverse effects, doctors have to limit the radiation dosage, which may reduce how effective the treatment is at fighting the cancer.
“[FLASH radiotherapy] offers the possibility of delivering larger doses of radiation, which could result in higher cure rates.”
John Breneman
The FLASH effect: FLASH radiotherapy is a promising alternative to traditional radiation therapy.
It delivers a dose of radiation that’s over 300 times higher than traditional radiation therapy in just a fraction of a second. This induces something called the “FLASH effect” — a not-entirely-understood phenomenon in which the radiation still attacks the tumor, but doesn’t harm surrounding tissue.
“This offers the possibility of delivering larger doses of radiation — which could result in higher cure rates for patients with resistant tumors — without increasing side effects,” said John Breneman, principal investigator of the new trial.
In animal studies, FLASH radiotherapy has been shown to be safe and just as effective as traditional radiation therapy without causing unexpected side effects. Now, a University of Cincinnati-led team has shared the results of FAST-01, the first-in-human trial of the treatment.
One FLASH radiotherapy treatment lasts just 0.3 seconds.
The trial: The primary goal of the trial was to prove that FLASH radiotherapy is safe for people and has a feasible workflow. A secondary goal was to determine its efficacy by measuring how much pain relief it provided patients.
The trial included 10 patients with painful cancerous growths in the bones of their arms or legs. Each received one FLASH radiotherapy treatment — lasting just 0.3 seconds — at the site(s) of their cancer.
Their pain, use of pain meds, and adverse effects were measured the day they received the therapy, 15 days later, and one, two, and three months after treatment.
“We did not see any unexpected additional toxicity with the substantially shorter treatment.”
Emily C. Daugherty
The results: Patients’ average time on the table was just 15.8 minutes per treated site — demonstrating that the workflow is feasible — and of the 12 total cancer sites treated, pain was fully relieved in six and partially relieved in two others.
“[B]oth pain relief and side effects were in-line with what might have happened with conventional radiation,” said lead author Emily C. Daugherty. “We did not see any unexpected additional toxicity with the substantially shorter treatment.”
Looking ahead: Ultimately, the researchers believe FLASH radiotherapy would be most useful for treating cancers in the brain, lungs, or gastrointestinal area, as the tissues around those tumors are particularly vulnerable to damage from traditional radiation therapy.
It could also be useful for treating cancers in children, who are more sensitive to the side effects of radiation therapy.
Since the limb treatments didn’t produce any unexpected side effects in people, they’ve begun enrolling patients in FAST-02, a trial that will target cancerous growths in the bones of the thorax, which surround the heart and lungs.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.