Researchers at Stanford University believe they’ve developed an effective and quick-acting technique to treat challenging cases of depression — using magnetic brain stimulation. The treatment, which is safe and non-invasive, could step in when other medications or treatments can’t do the job.
Why this matters: Depression is the leading cause of disability globally. And, as much as half of the people with depression have treatment-resistant depression. With suicide being among the leading causes of death in the United States, finding a reliable, fast way to treat depression is critical.
Trending: The practice of using brain stimulation to treat depression is still new, and many methods aren’t yet proven. But many researchers think they could help people with depression. The idea is that when antidepressants or psychotherapy can’t help the most severe cases of depression, electrical impulses can. The impulses compensate for irregular brain activity, which may be causing depression.
The team found that with insight from an MRI scan, they can personalize the brain stimulation treatment and cut the time down to a few days.
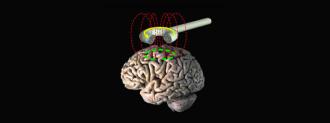
There are a variety of types of brain stimulation, some are invasive and require a brain implant while others are non-invasive. The type used in this new study is a modified version of transcranial magnetic stimulation (rTMS), which is a non-invasive procedure that uses a magnetic coil placed near the skull to send pulses into the brain.
What they did: Typically rTMS treatment takes several weeks. But the team from Stanford found that with insight from an MRI scan, they can personalize the brain stimulation treatment and cut the time down to a few days, and in some cases, even just one treatment day made a difference.
By using MRI scans, the team was able to pinpoint the best places on the brain to deliver the pulses to each patient. The Stanford method (dubbed Stanford neuromodulation treatment or SNT) also uses stronger impulses in a shorter amount of time than standard rTMS treatments — 5 days of SNT is roughly equivalent to 7 months of standard rTMS treatment, according to Gizmodo.
To test their new method, the team recruited 29 volunteers who had treatment-resistant depression, meaning their depression could not be treated by other methods like therapy or SSRIs. Half of the group received the real SNT procedure, complete with magnetic impulses. The other half received a sham treatment. Then the researchers monitored the entire group for four weeks.
After the monitoring period had passed, the participants went through a round of standard diagnostic tests. The team found that 78% of the patients who received this new treatment benefited. The treatment reduced symptoms of their depression by 52% — according to the results of a standardized diagnostic questionnaire that psychologists use to measure depression.
Whereas only 13% of the people who received the placebo treatment felt relief in their depression.
“It works well, it works quickly and it’s noninvasive,” Nolan Williams said in a statement. They published their study in the American Journal of Psychiatry.
The FDA approved rTMS in 2008, but the results have been touch and go. Previous research found that only about 30% at best will experience remission. So, the treatment was used only when other treatments didn’t work. But, Stanford’s version of rTMS has an overwhelmingly better success rate. And, in severe cases of severe depression, where people could be suicidal, cutting down the time it takes to ease the suffering is critical. SNT could be a lifesaver.
With the degree of success from their initial study, the team believes they have a real option for treating severe depression. Williams called it a “gamechanger.” They say it could even outperform electroconvulsive therapy, a frequently used brain stimulation method for treatment-resistant depression. They’ve already received patents for SNT and are currently seeking FDA approval.
“We were pleasantly surprised that this was more powerful, statistically, than what we anticipated. And then we felt it important to stop the trial and get the information out there as soon as we could,” Williams told Gizmodo.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.