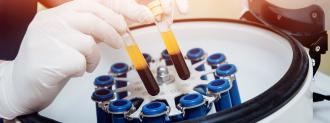
Two separate studies found that patients with severe cases of COVID-19 benefited from transplants of blood plasma from coronavirus survivors.
This form of treatment, known as convalescent plasma therapy, has been around for more than a century, ever since doctors used blood transfusions from Spanish flu survivors to effectively treat patients still battling the illness.
However, these small studies are some of the first evidence that the treatment could be an effective weapon against the novel coronavirus — and more proof could be just on the horizon.
Plasma from Coronavirus Survivors
After a person recovers from an infection, their blood plasma contains antibodies that alert their immune system to attack the virus or bacteria if it ever shows up again.
By transplanting plasma from coronavirus survivors into the bodies of people still battling the infection, doctors hoped those valuable antibodies would help their immune systems clear it — and based on the results of these two studies, that appears to be the case.
Within three days of treatment, the patients’ coronavirus symptoms significantly improved.
For one of the studies, published in the journal Proceedings of the National Academy of Sciences, doctors in Wuhan, China, treated 10 severely ill COVID-19 patients with one dose of plasma from coronavirus survivors.
Within three days, the patients’ coronavirus symptoms — fever, cough, shortness of breath, and chest pain — “significantly improved.” Their oxygen levels also improved, and the treatment appeared to speed up the clearance of the virus from their bodies.
The other study, published in the Journal of the American Medical Association, involved five severely ill coronavirus patients in Shenzhen, China.
All of those patients showed improvement after receiving plasma from coronavirus survivors. Within 10 days, three no longer needed to be on ventilators, and 37 days after the transfusion, two were in stable condition and three had already left the hospital.
Combatting COVID-19
With just 15 patients between them, these two trials are far too small to prove that convalescent plasma therapy is a safe and effective treatment for COVID-19.
However, researchers have yet to find a drug treatment for the novel coronavirus, and we likely won’t have a coronavirus vaccine for at least a year.
That means any encouraging lead in the fight against COVID-19 is worth pursuing — and that’s exactly what researchers are now doing with convalescent plasma therapy.
On April 3, the U.S. Food and Drug Administration announced that it was launching a nationwide trial to explore the safety and efficacy of convalescent plasma therapy as a coronavirus treatment.
The Mayo Clinic is serving as the lead research institute for that trial, with the American Red Cross handling the procurement and distribution of plasma from coronavirus survivors.
“The FDA anticipates that this collaborative effort will be able to move thousands of units of plasma to the patients who need them in the coming weeks,” the agency wrote in a statement.
That means we could know very soon whether the promise of the Chinese trials holds up when applied to a far larger patient sample — and whether the treatment could effectively minimize the impact of COVID-19 while we wait for a vaccine to emerge.