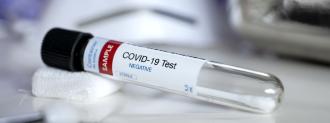
On April 21, the U.S. Food and Drug Administration granted emergency use authorization to the nation’s first at-home coronavirus test.
According to the FDA, LabCorp’s self-administered COVID-19 test is just as accurate and safe as those done by healthcare professionals — and it could have a number of benefits over existing diagnostics methods.
LabCorp’s At-Home Coronavirus Test
The first step to buying one of LabCorp’s at-home coronavirus test kits is completing an eligibility survey on the company’s website, which asks about symptoms, contact with the virus, and vulnerability to it. Also, because supplies are limited, they are prioritizing tests for healthcare workers for now.
The company provides access to test results online one to two days after a sample arrives at their lab.
Eligible people can then order one of the kits for $119. Buyers must pay upfront, but can seek reimbursement from their insurance provider later. An independent physician will review their request before the kit ships via FedEx.
Once a person has the kit in hand, they use a nasal swab to collect their own specimen sample from the inner edges of their nostrils — no need for the painfully deep swab favored by most early COVID-19 tests.
The person then places the swab into an insulated package and mails it to a LabCorp lab for analysis. The company says they should be able to access their results online one to two days after the sample arrives at the lab.
Staggered Rollout
The FDA authorization doesn’t mean everyone in the U.S. will suddenly have access to an at-home coronavirus test, however.
At first, LabCorp is only going to sell the tests to healthcare workers and first responders who are symptomatic or have been exposed to the coronavirus.
LabCorp plans to make the kits available to the general public in the coming weeks.
According to a news release, it plans to make the kits available to the general public in “coming weeks.”
Even then, though, residents of New York, New Jersey, Maryland, and Rhode Island will not be allowed to buy the kits due to state regulations that prohibit patients from initiating their own laboratory tests, LabCorp spokesperson Mike Geller told CBS News.
That’s unfortunate as New York and New Jersey currently have more coronavirus cases than any other states in the U.S.
The Benefits of At-Home Testing
One of the biggest hurdles to effectively combating the COVID-19 outbreak in the U.S. has been securing enough tests.
That makes the authorization of any new coronavirus test good news — and the fact that LabCorp’s test allows patients to collect their own specimen samples is even better.
For one, putting the specimen-collection process in patients’ hands — and not those of medical staff — could decrease demand for personal protective equipment.
An at-home coronavirus test could also increase testing among populations currently incapable of reaching medical clinics. People who live in remote areas, for example, or those with inadequate transportation will be able to mail in their self-collected samples for analysis.
One of the biggest potential benefits, though, is that the at-home coronavirus test could help minimize the spread of the virus.
Once the test is more widely available, many people who have the virus will no longer need to venture out of their homes — and to places where they might infect others — for diagnostics testing.
Those who don’t have the virus, meanwhile, won’t have to take the risk of going to a medical office or clinic — where they might catch it — just to find out they’re infection-free.